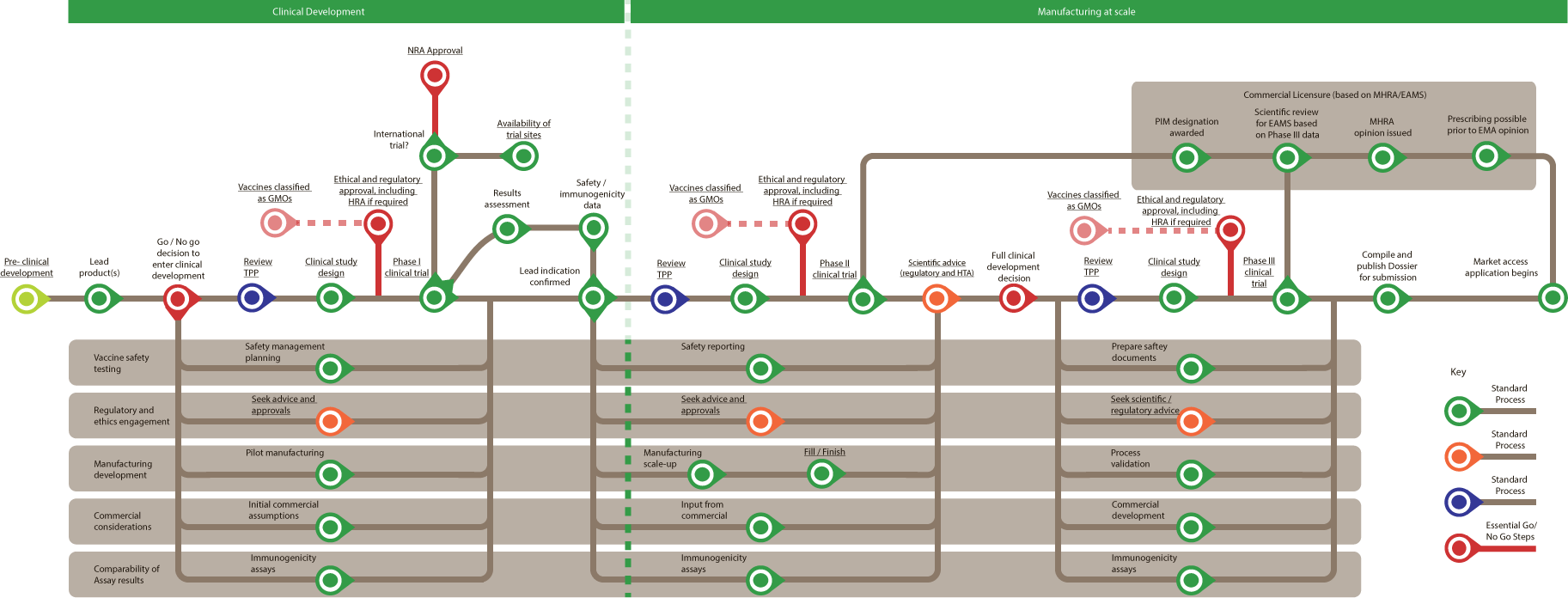
Clinical Development
Bottlenecks in the process are marked with a B.
All are standard proces unless stated otherwise.
Clinical Development
- Lead products
- Go/No go decision to enter clinical development (Essential Go/No Go step)
- Vaccine safety testing – Safety management planning
- Regulatory and ethics engagement – Seek advice and approvals
- Manufacturing – Pilot manufacturing
- Commercial considerations – Initial commercial assumptions
- Comparability of Assay results – Immunogenicity assays
- Review TPP
- Clinical study design
- Required at this stage -
- Ethical and regulatory approval, including HRA if required (Essential Go/No Go step) B
- Vaccines classified as GMOs (Essential Go/No Go step) B
- Phase 1 clinical trial
- International trial?
- Availability of trial sites B
- NRA Approval (Essential Go/No Go step)B
- Results assessment
- Safety/ immunogenicity data
- Lead indication confirmed
- Vaccine safety testing – Safety reporting
- Regulatory and ethics engagement – Seek advice and approvals
- Manufacturing – Manufacturing scale-up
- Commercial considerations – Input from commercial
- Comparability of Assay results – Immunogenicity assays
- Review TPP
- Clinical study design
- Required at this stage -
- Ethical and regulatory approval, including HRA if required (Essential Go/No Go step) B
- Vaccines classified as GMOs (Essential Go/No Go step) B
- Phase II clinical trial
- Commercial Licensure )based on MHRA/EAMS) – PIM designation awarded
- Commercial Licensure )based on MHRA/EAMS) – Scientific review for EAMS based on Phase III data
- Commercial Licensure )based on MHRA/EAMS) – MHRA opinion issued
- Commercial Licensure )based on MHRA/EAMS) – Prescribing possible prior to EMA opinion
- Scientific advice (Regulatory and HTA)
- Full clinical development decision (Essential Go/No Go step)
- Ongoing from this point –
- Vaccine safety testing – Prepare safety documents
- Regulatory and ethics engagement – Seek scientific/regulatory advice
- Manufacturing – Process validation
- Commercial considerations – Commercial development
- Comparability of Assay results – Immunogenicity assays
- Review TPP
- Clinical study design
- Required at this stage -
- Ethical and regulatory approval, including HRA if required (Essential Go/No Go step) B
- Vaccines classified as GMOs (Essential Go/No Go step) B
- Phase III clinical trial
- Compile and publish Dossier for submission
- Market access application begins
(A to E ongoing until after Phase 1 clinical trial – Step 6 below)
(A to E ongoing until Scientific advice – Step 14 below)
Manufacturing at scale
(A to D ongoing in parallel with Steps 9 to 18 until Market access application begins – Step 18 below)
(A to E ongoing until after Phase III clinical trial – Step 16 below)
End of Process
![]() Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.
Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.