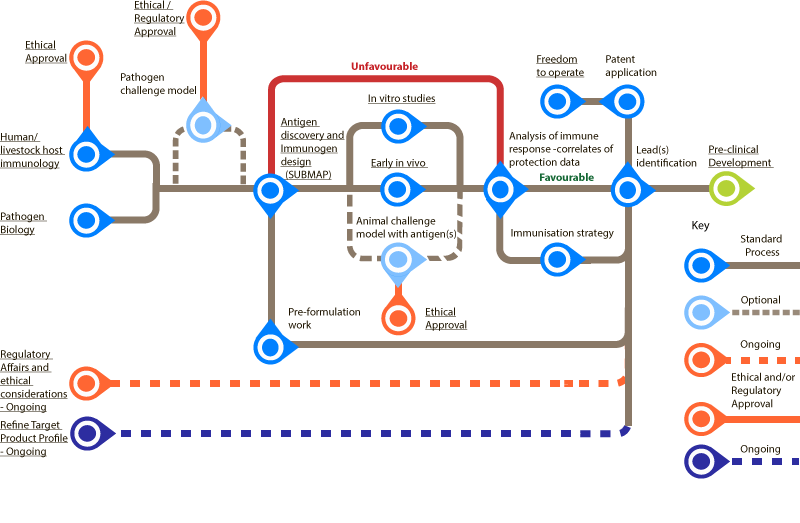
Pre-Clinical Discovery
Bottlenecks in the process are marked with a B.
All are standard proces unless stated otherwise.
- Human/livestock host immunology B
- Pathogen Biology B
- Pathogen challenge model (Optional) B
- Antigen discovery and immunogen design
- Vaccine type
- Whole microbial
- Subunit, vectored, glycoconjugate
- Antigen selection and purification (Optional)
- Growth rate, Manufacture, Safety
- Antigen/gene of interest, vaccine vector, adjuvant
- Proceed to in vitro/in vivo studies
- Pre-formulation work
- In vitro studies
- Early in vivo
- Animal challenge model with antigen(s) (Optional)
- Analysis of immune response – correlates of protection data (If favourable continue to Steps 10 and 11, if unfavourable go back to Step 4)
- Immunisation strategy
- Lead(s) identification
- Freedom to operate B
- Patent application
Ongoing throughout process:
![]() Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.
Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.