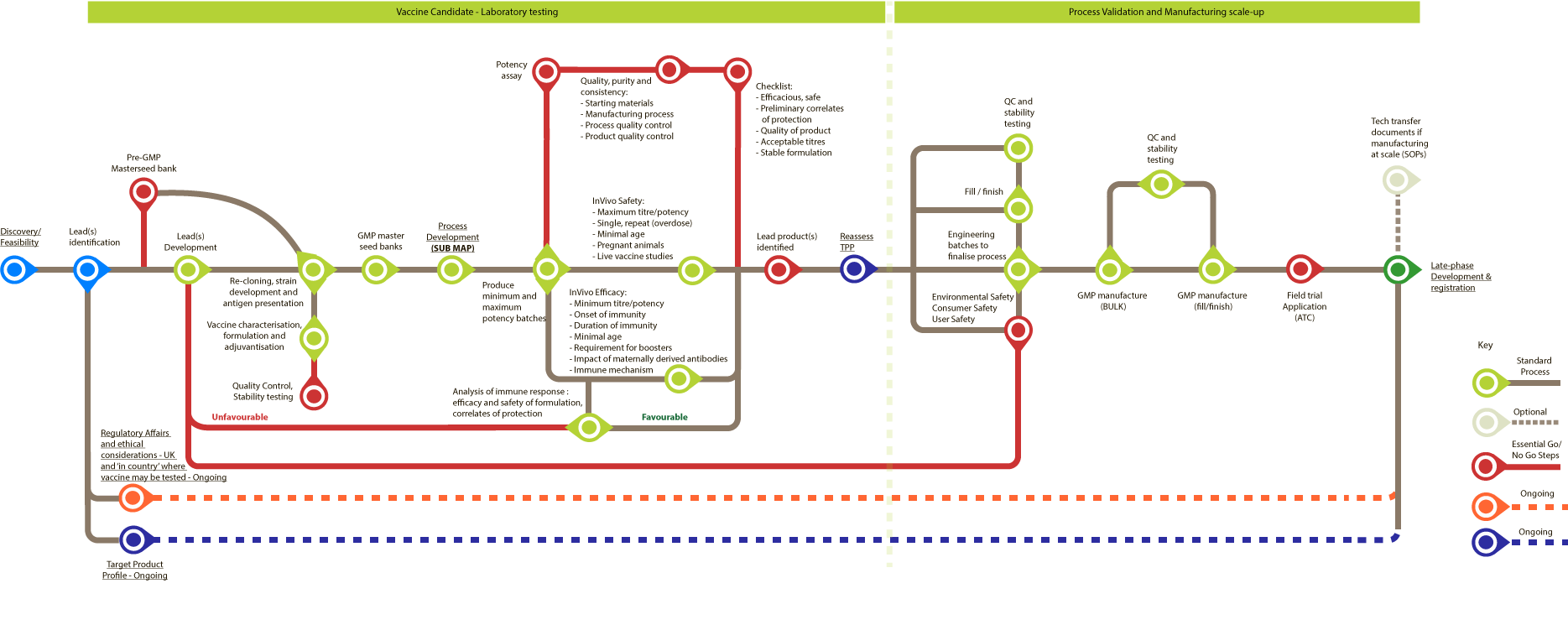
Veterinary Vaccine: Early-phase Development
Bottlenecks in the process are marked with a B.
All are standard proces unless stated otherwise.
Vaccine candidate – laboratory testing
- Lead(s) identification
- Pre-GMP Masterseed bank (essential go/no go step) B
- Lead(s) Development
- Re-cloning, strain development and antigen presentation B
- Vaccine characterisation, formulation and adjuvantisation B
- Quality Control, Stability testing (essential go/no go step)
- GMP master seed banks B
- Process development B
- Process Design
- Process Analytics (1. In process controls, 2. Product/Impurities Characterisation, 3. Release Assays (QA/QC) )
- Process Flowsheet and optimisation (1. Process Flow sheet, 2. Critical Process Parameters (CPP)/Critical Quality Attributes (CQA)s, 3. SOPs and sample data, 4. Master Seed Bank transfer and testing)
- Pilot scale-up studies
- Use of pilot lots in developmental studies
- Produce minimum and maximum potency batches B
- Potency assay (essential go/no go step)
- Quality, purity and consistency: Starting materials, Manufacturing process, Process quality control, Product quality control (essential go/no go step) B
- Checklist: Efficacious, Prelliminary correlates of protection, Quality of product, Acceptable titres, Stable formulation (essential go/no go step)
- In vivo safety: Maximum titre/potency, Single, repeat (overdose), Minimal age, Pregnant animals, Live vaccine studies B
- In vivo efficacy: Minimum titre/potency, Onset of immunity, Duration of immunity, Minimal age, Requirement for boosters, Impact of maternally derived antibodies, Immune mechanism B
- Analysis of immune response: efficacy and safety of formulation, correlates of protection. (If favourable continue to Step 12, if unfavourable go back to Step 3)
- Lead product(s) identified (essential go/no go step)
- Reassess TPP
- QC and stability testing
- Fill/finish
- Engineering batches to finalise process
- Environmental safety, Consumer safety, User safety (essential go/no go step) (If unfavourable go back to Step 3)
- GMP manufacture (BULK) B
- QC and stability testing
- GMP manufacture (fill/finish) B
- Field trial Application (ATC) (essential go/no go step)
- Late-phase Development and registration
- Tech transfer documents if manufacturing at scale (SOPs, TPP) (optional)
The following steps 8 to 11 are in parallel.
Process Validation and Manufacturing scale-up
The following steps 14 to 17 are in parallel.
Ongoing throughout process:
- Regulatory Affairs and ethical considerations - UK and 'in country' where vaccine may be tested
- Target product profile
Next list view of map – Late-phase Development and Registration Map
![]() Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.
Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.