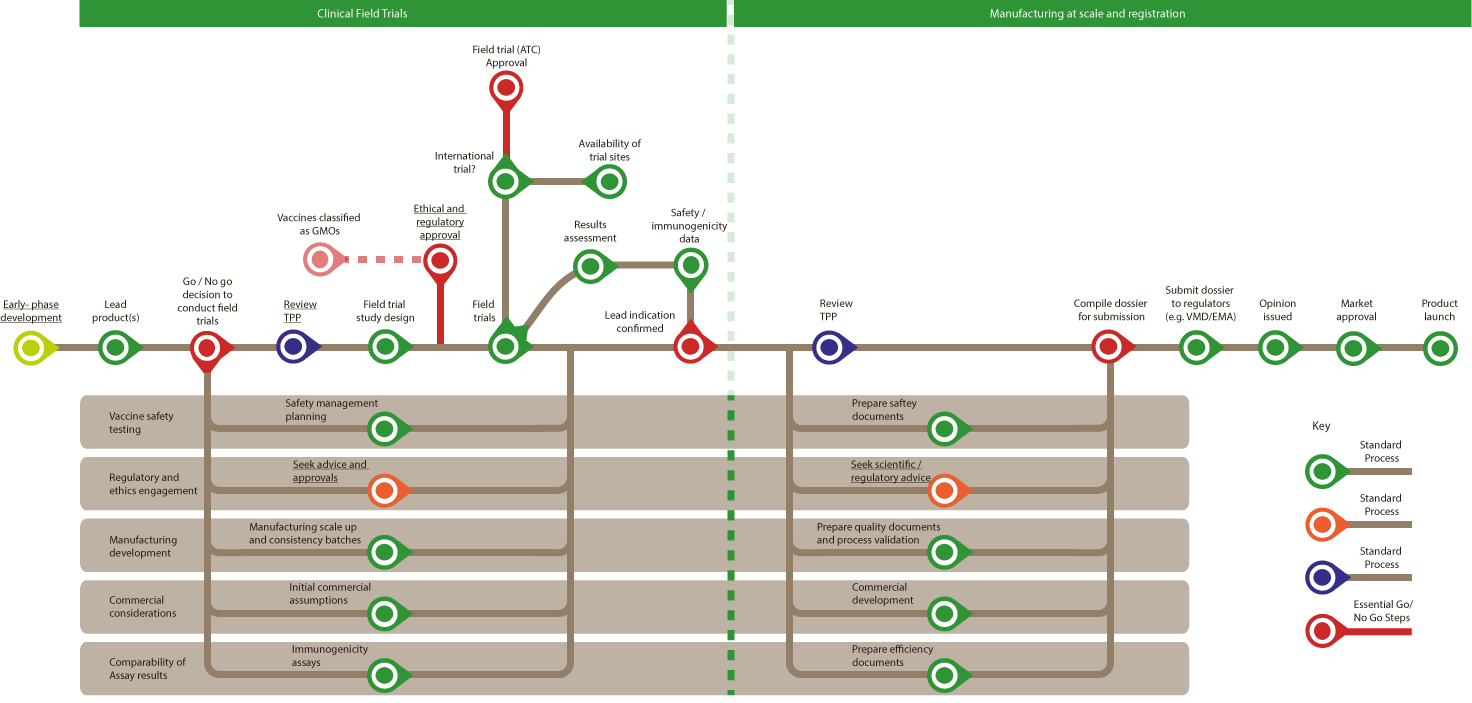
Veterinary Vaccine: Late-phase Development and Registration
Bottlenecks in the process are marked with a B.
All are standard proces unless stated otherwise.
Clinical Field Trials
- Lead(s) products
- Go/No go decision to enter conduct field trials (Essential Go/No Go step)
- Vaccine safety testing – Safety management planning
- Regulatory and ethics engagement – Seek advice and approvals
- Manufacturing development – Manufacturing scale up and consistency batches
- Commercial considerations – Initial commercial assumptions
- Comparability of Assay results – Immunogenicity assays
- Review TPP
- Field study design
- Required at this stage -
- Ethical and regulatory approval (Essential Go/No Go step) B
- Vaccines classified as GMOs (Essential Go/No Go step) B
- Field trials
- International trial?
- Availability of trial sites B
- Field trial (ATC) Approval (Essential Go/No Go step)B
- Results assessment
- Safety/ immunogenicity data
- Lead indication confirmed
- Ongoing through steps 9 to 10
- Vaccine safety testing – Prepare safety documents
- Regulatory and ethics engagement – Seek scientific/regulatory advice
- Manufacturing development – Prepare quality documents and process validation
- Commercial considerations – Commercial development
- Comparability of Assay results – Prepare efficiency documents
- Review TPP
- Compile dossier for submission (Essential Go/No Go step)
- Submit dossier to regulators (e.g. VMD/EMA) B
- Opinion issued
- Market approval
- Product launch
(A to E ongoing until after Field trials – Step 6 below)
Manufacturing at scale and registration
End of Process
![]() Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.
Underlined nodes have further information and can be selected. Those using assistive technologies can access the full information using the list view of the map.